Kaffes™ : A Game-Changer in Resolving Post-Liver Transplant Biliary Anastomic Strictures
20/09/2024
Abstract
Background
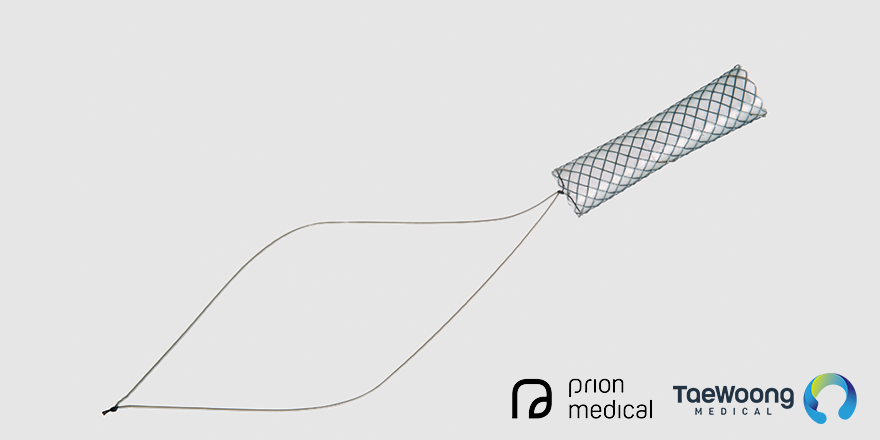
We report our experience of treating anastomotic strictures using a novel type of fully covered metal stent (FCSEMS). This stent, known as the Kaffes Stent, is short-length with an antimigration waist and is easily removable due to long retrieval wires deployed within the duodenum.
Methods
Sixty-two patients underwent ERCP and Kaffes stent insertion for post-transplant anastomotic strictures following confirmation of a stricture on MRCP. These patients were retrospectively analysed for immediate and long-term stricture resolution, improvement in symptoms and liver function tests (LFTs), stricture recurrence and complication rates.
Results
Of the 56 patients who had their stent removed at the time of analysis, 54 (96%) had immediate stricture resolution and 42 continued to have long-term resolution (mean follow-up period was 548 days). Of the 16 patients with symptoms of biliary obstruction, 13 had resolution of their symptoms. Overall, there was a significant improvement in LFTs after stent removal compared to before stent insertion. Complication rates were 15% with only one patient requiring biliary reconstruction.
Conclusions
The Kaffes stent is effective and safe at resolving post liver transplant biliary anastomotic strictures.
Warner, B., Harrison, P., Farman, M. et al. A unique type of fully covered metal stent for the management of post liver transplant biliary anastomotic strictures. BMC Gastroenterol 20, 329 (2020). https://doi.org/10.1186/s12876-020-01479-6